Iodine and Natural Gas
Iodine
Properties of Iodine -- What is Iodine?
Iodine is an element well-known for its color-changing reaction with starch to produce a purple black color (Iodine-starch reaction), or as a nutrient found in kelp and other seaweed. Since iodine is an element, it cannot be manufactured by means of chemical synthesis. We can obtain iodine only by extracting it from iodine-containing natural resources. That’s why iodine is precious. Commercially-available iodine is a heavy solid, just like a metal, with a purple-black metallic luster. Like dry ice, it easily sublimes (evaporates) even at ordinary temperature, producing a peculiar odor.
Use of Iodine -- Why is Iodine Necessary?
Iodine is essential to our life because it is processed into various compounds and used in many areas of our life for its strong reactivity (oxidizability).
Iodine is also an essential element in human growth. The daily iodine requirement is said to be 150μg.
Principal use of iodine
- 1.
Introduced as a substituent group into an enhancing agent for medical diagnostic X-rays for its excellent X-ray absorption capacity. Several medicinal or agricultural chemicals have iodine substituents.
- 2.
Used as a disinfectant with antimicrobial effect originating from elemental iodine.
- 3.
Contained in dietary salt and feeding stuff as an iodine preparation to treat or prevent iodine deficiency.
- 4.
- Used for a wide range of industrial purposes including photosensitive materials, catalysts, stabilizers and polarizing films for liquid crystal display panels. Iodine compounds are widely used as reactive intermediates for their high reactivity.
Location of Iodine -- Where Can We Find Iodine?
Iodine is contained in seawater, seaweed, brine and a mineral (niter) in the form of iodide ion or iodine compounds. Since their iodine content is very low, the areas on earth where iodine is economically obtainable are extremely limited. Annual worldwide production of iodine is roughly 18,000 tons. Chile, Japan, and other areas including USA account for 50%, 40% and 10% of global iodine production, respectively. In Japan, Chiba prefecture is the largest iodine producer, and the source of iodine is brine pumped up from the deposits of natural gas dissolved in water beneath Chiba prefecture. Therefore, iodine is a noteworthy precious resource in resource-poor Japan.
What are the Deposits of Natural Gas Dissolved in Water? Why are They Iodine-rich?
Deposits of natural gas dissolved in water beneath Chiba prefecture form a salt-rich groundwater layer contained together with natural gas in the stratum formed 2 to 12 million years ago. The groundwater is called brine. Seaweed (which is rich in iodine, and iodine was extracted from seaweed ash in olden days), and other organic substances deposited together with soil on the ancient seabed, are said to form the groundwater layer. It is thought that iodine contained in seaweed and other substances had been concentrated over a long period of time, and became a source of today’s iodine. Brine has a similar saline concentration to seawater. Meanwhile, the iodine level in brine is around 100ppm, almost 2,000 times higher than that of seawater. Places where iodine is naturally concentrated like this are rarely found in the world. As a result, this area accounts for nearly 40% of global iodine output. Our company has its own mining area, and produces brine and gas.

Natural Gas
Properties of Natural Gas -- What is Natural Gas?
It is well known that what is generally called natural gas is a naturally occurring inflammable gas of which the chief components are hydrocarbons, and that natural gas is produced in oil-field regions and imported in vast quantities to Japan in the form of LNG (liquid natural gas). LNG is dissolved gas occurring with petroleum oil. It is a mixture of methane, ethane, propane, butane, etc. The natural gas used by our company is what is called natural gas dissolved in water. It occurs with brine from deposits of natural gas dissolved in water, which are referred to as the Southern-Kanto Gas Field distributed in a sedimentary basin extending beneath Chiba to Ibaraki, Saitama, Tokyo and Kanagawa prefectures. Our company has its own mining area (about 28 square kilometers), and produces gas and brine. The natural gas we produce is almost pure methane. Since it does not have any sulfur content, it is used as clean energy for fuels including city gas. Or it is used as a chemical raw material without purification.
Our company uses the gas we pump out not only as fuel and for selling to city gas suppliers, but also as a synthetic raw material for hydrogen cyanide.
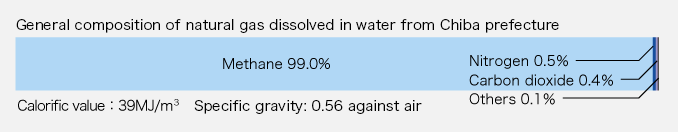
Location of Natural Gas -- Where Can We Find Natural Gas?
Deposits of natural gas dissolved in water are distributed widely in Japan, including Hokkaido, Akita, Niigata, Chiba, Shizuoka, Miyazaki and Okinawa prefectures. Among them, the Southern-Kanto Gas Field is the largest both in output and reserves. Especially, the deposits spreading beneath Chiba prefecture are abundant in iodine, which is produced from brine pumped out together with natural gas.
Origin of Natural Gas -- How is Natural Gas Formed?
Deposits of natural gas dissolved in water spreading beneath Chiba prefecture form a salt-rich groundwater layer contained together with natural gas in the stratum formed 2 million years ago. The groundwater is called brine. Seaweed, and other plants and animals deposited together with soil on the ancient seabed from the late Pliocene to the middle Pleistocene eras (start of ice age and appearance of human beings), are presumed to have formed the groundwater. Natural gas dissolved in water is methane gas formed by microbial decomposition of the organic substances buried in the earth. The deposited material is thought to contain large amounts of iodine-rich seaweeds. These organic substances, after breaking down over a long period of time, dissolved and accumulated little by little in underground water. The resultant water containing concentrated breakdown products is thought to be the groundwater currently found in the deposits of natural gas dissolved in water. The groundwater is present in spaces between sandy mud grains in the stratum called the Kazusa Group which consists of alternating layers of sandstones and mudstones, and lies about 200 to 2,000m below the surface. Under the ground, natural gas is dissolved in the groundwater (brine) due to the high pressure. When it is pumped up to the surface, freed natural gas separates from brine. Brine has a similar saline concentration to seawater. Meanwhile, the iodine level in brine is around 100ppm, almost 2,000 times as high as that in seawater. Places where iodine is naturally concentrated like this are rarely found in the world. That’s why iodine is a noteworthy precious resource in resource-poor Japan.
How to Produce Iodine
Our company manufactures iodine by the blowing-out method. This manufacturing method utilizes the property of iodine to readily vaporize. The iodine content in brine as iodide ion (I-) is relatively low, around 100ppm. This method is suitable to extract iodine (I2) from such dilute aqueous solutions. After removing sand and impurities in the brine by precipitation, an oxidizer is added to liberate the iodine (I2), which is then blown out of the brine with air. The blown-out iodine (I2) is then absorbed, crystallized and purified to manufacture commercially-available iodine. This method has been used by many plants in the USA and Japan. Our company improves the iodine recovery rate from brine through our technology and experience accumulated over 50 years and endless efforts for improvement, and enjoys high manufacturing efficiency.
Recent Patents:Japanese Patents Nos.2732635, 2732636, 2732637, 2732642

Iodine Recycling Technology
Elemental iodine or iodine compounds are used in manufacturing various industrial products. Then, iodine is often eliminated, rather than remaining in the products. Due to its high reactivity, iodine compounds are often used as reaction intermediates. After the reaction, iodine itself is not introduced in the target compound, and is removed from the reaction system. Eliminated iodine is a sort of industrial waste after manufacture of an industrial product. It is commonly known as “Recovered Iodine”, and remains in the form of aqueous solution or solid. Recovered iodine contains various inorganic and organic substances other than iodine. If it is released to the environment as it is, it would not only become an environmental burden but also be a waste of precious iodine. This could be a large loss. Accordingly, our company actively engages in iodine recovery and recycling from the viewpoint of environmental and resource protection.
There are various forms of recovered iodine, and iodine content and components other than iodine vary a lot. Our company gives appropriate pretreatment suitable for each form of iodine based on the technology we developed and experience we accumulated for re-commercialization of the recovered iodine.
Recent Patents:Japanese Patents Nos. 2539858, 2575152, 4674168, 4758766

How to Synthesize Hydrogen Cyanide
Our company synthesizes hydrogen cyanide from pumped natural gas (methane) by the Andrussow method, and utilizes it in cyanation reactions. We handle liquid hydrogen cyanide safely by the technologies we have developed over the years.
Properties of Hydrogen Cyanide
HCN |
|||
|---|---|---|---|
Chemical formula |
HCN |
Specific gravity |
d20 0.688 |
Molecular weight |
27.03 |
Melting point |
-13.3℃ |
Boiling point |
25.7℃ |
||
It is well known that hydrogen cyanide is formed as a byproduct of acrylic nitrile, and hydrogen cyanide is neutralized with sodium hydroxide immediately after its formation to manufacture cyanide of sodium (sodium cyanide). As you know, sodium cyanide is a chemical essential in plating and other industries, as typified by plating applications, and distributed in large quantities. As a useful and essential chemical compound, cyanogen compounds (commonly called cyanides) are used in a wide range of industries under strict control in conformity with laws and regulations. Our company takes advantage of having a natural gas (methane gas) field, and synthesizes hydrogen cyanide from methane. Synthesized hydrogen cyanide is used in various cyanation reactions as liquid hydrogen cyanide.
Cyanide Disposal Technology
Generation of cyanide-containing wastewater is inevitable in manufacturing of cyanides. Without eliminating cyanogen completely, the waste water cannot be discharged to the environment. In Chiba prefecture, especially stringent standards are imposed on the effluent water, so that no cyanogen is detected.
We are keenly aware of our responsibility as manufacturer and user of cyanides, pay especially close attention to cyanide disposal, and have established a system whereby cyanogen is not discharged into the environment. Cyanide-containing wastewater is treated by a method suitable for the nature of the waste water, and other hazardous substances are also eliminated to make the waste water meet effluent standards. Organic cyanogen wastewater, whose chemical treatment is especially difficult, is subjected to thermal disposal at 1,000 degrees C using pumped natural gas as fuel. This dioxin-free process can eliminate cyanides and other organic components completely.








